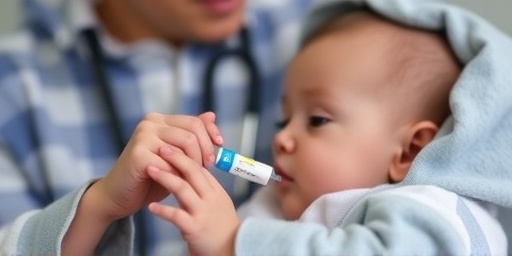
In a critical move to combat the escalating RSV winter surge, the U.S. Food and Drug Administration (FDA) has granted emergency use authorization for nirsevimab, a groundbreaking monoclonal antibody preventive designed specifically for high-risk infants. This decision comes as pediatric hospitals across the country report unprecedented spikes in respiratory syncytial virus (RSV) cases, with hospitalizations surging by over 60% in states like Texas, Florida, and California compared to last year.
The authorization, announced late Friday, targets infants under 8 months entering their first RSV season and certain high-risk toddlers up to 24 months. Unlike traditional vaccines, nirsevimab—branded as Beyfortus and developed by AstraZeneca and Sanofi—provides immediate, long-lasting protection with a single dose, filling a vital gap in pediatric care during this brutal winter surge.
Health officials emphasize that this approval couldn’t come soon enough. The CDC reports over 14,000 RSV-related hospitalizations in infants nationwide in the past month alone, straining emergency rooms and leading to pediatric bed shortages in major cities.
RSV Hospitalizations Hit Record Highs Across Key States
The RSV winter surge has transformed hospitals into battlegrounds for pediatric care. In Texas, where RSV cases have exploded by 75% year-over-year, facilities like Texas Children’s Hospital in Houston have seen daily admissions double, with ICUs at 95% capacity. Dr. Maria Gonzalez, a pediatric infectious disease specialist at the hospital, shared, “We’ve never seen anything like this. Infants are arriving in droves, many requiring oxygen or ventilation. The winter surge is overwhelming our system.”
Florida reports similar chaos, with the Florida Department of Health logging a 50% increase in RSV-positive tests among infants under 6 months. Miami Children’s Hospital diverted non-emergency cases last week after RSV admissions hit 200—a record. California isn’t spared; Los Angeles County alone saw 1,200 infant RSV hospitalizations in November, up 40% from 2022 peaks.
Nationwide, the American Academy of Pediatrics (AAP) warns of a “tripledemic” threat from RSV, flu, and COVID-19. CDC data shows RSV now accounts for 70% of pediatric respiratory hospitalizations, with infants under 6 months facing the highest risk—up to 2-3% hospitalization rates per season. Last winter’s surge hospitalized 58,000 children under 5, but experts predict this year could exceed 80,000 without interventions like the new FDA-approved preventive.
Contributing factors include waning maternal antibodies post-summer, crowded daycare settings, and low vaccination rates for maternal RSV shots like Pfizer’s Abrysvo. Wastewater surveillance from Biobot Analytics confirms RSV viral loads at all-time highs in 20 major metros.
Breakdown of FDA’s Emergency Nod for Nirsevimab Preventive
The FDA‘s emergency use authorization for nirsevimab marks a pivotal advancement in RSV prophylaxis. Administered as a single intramuscular injection, the monoclonal antibody mimics natural antibodies to neutralize the RSV F protein, preventing viral entry into lung cells. Clinical trials involving over 3,000 infants demonstrated 74.5% efficacy against medically attended lower respiratory infections and a remarkable 78% reduction in RSV hospitalizations.
Unlike palivizumab (Synagis), the prior standard requiring monthly doses, nirsevimab offers six-month protection from one shot, slashing administration costs by 50-70%. Priced at around $550 per dose, it’s covered under the Vaccines for Children program for eligible families. The FDA reviewed data from the MELODY and HARMONIE trials, where adverse events were mild—mostly injection-site reactions—and no new safety signals emerged.
“This is a game-changer for high-risk infants,” said FDA Commissioner Dr. Robert Califf in a statement. “Emergency authorization accelerates access during this RSV winter surge, potentially saving thousands of hospitalizations.” The green light applies to infants born during or entering RSV season (October-March) and those with conditions like prematurity, congenital heart disease, or chronic lung issues.
Supply chains are ramping up; AstraZeneca projects 1 million doses available by January, prioritizing high-burden areas. Pediatricians can administer it in outpatient settings, easing hospital loads.
Pediatric Experts and Parents React to Timely FDA Approval
Frontline clinicians are hailing the FDA decision as lifesaving. “Finally, a tool that matches the threat,” tweeted Dr. Sean O’Leary, AAP immunization expert. “Nirsevimab could avert 40,000 infant hospitalizations this season alone.” A survey by the Pediatric Infectious Diseases Society found 92% of members plan immediate adoption.
Parents of high-risk infants express relief mixed with urgency. Sarah Jenkins, mother of a 4-month-old preemie in Atlanta, told reporters, “RSV terrifies me—my baby was in NICU last year. Knowing this RSV vaccine-like preventive is available now gives us hope amid the winter surge.” Support groups like RSV Survivors Network reported a 300% spike in queries post-announcement.
Critics, however, urge caution. Some bioethicists question expanding use beyond trials, citing rare anaphylaxis risks (0.05%). Insurers like UnitedHealthcare pledged coverage but warned of access disparities in rural areas, where 20% of U.S. infants live.
CDC Director Dr. Mandy Cohen added, “We’re coordinating with states for equitable distribution. This FDA approval aligns with our RSV prevention strategy, including maternal vaccination.” Quotes from Sanofi CEO Paul Hudson underscored commitment: “We’ve invested $1.5 billion; now it’s about delivery.”
Unpacking RSV Dangers for Infants and Protection Options
RSV, a common respiratory virus, hits infants hardest, causing bronchiolitis and pneumonia. Globally, it kills 100,000-200,000 children under 5 annually; in the U.S., it’s the leading cause of infant hospitalization. Symptoms start mild—runny nose, cough—but escalate to wheezing, apnea, and respiratory failure in 1-2% of cases, especially premies (40-50% risk) or those with neuromuscular disorders.
This winter surge is amplified by post-pandemic immunity debt and El Niño weather patterns boosting indoor transmission. Prevention has evolved: Maternal RSV vaccines like Abrysvo (approved May 2023) protect via antibodies in breast milk, but uptake lags at 20%. Nirsevimab complements this, recommended by AAP for all infants under 8 months unless maternally vaccinated.
- High-risk groups: Premature infants (<35 weeks), bronchopulmonary dysplasia, hemodynamically significant heart disease.
- Symptom watch: Fever, poor feeding, rapid breathing (>60 breaths/min), grunting.
- Home tips: Handwashing, avoiding sick contacts, masking in crowds.
Comparative efficacy: Nirsevimab outperforms Synagis (50% reduction) and pairs with flu/COVID shots for bundled protection.
Rollout Roadmap and Projected Relief from RSV Surge
As distribution accelerates, the FDA-backed nirsevimab rollout targets peak RSV months. ACIP unanimously endorsed universal use last summer; states like New York and Illinois are pre-positioning doses in clinics. AstraZeneca’s U.S. manufacturing ramp-up ensures 500,000 doses by mid-December, with global expansion eyed for 2024.
Modelers from Johns Hopkins forecast a 30-50% drop in infant RSV hospitalizations if 70% coverage is achieved, easing $2 billion annual costs. Long-term, full FDA approval (expected Q2 2024) and competition from Moderna/GSK RSV vaccines could normalize prevention.
Challenges remain: Vaccine hesitancy (25% parental refusal per polls), supply chain hiccups, and monitoring resistance. Yet, officials project this intervention curbs the winter surge, buying time for herd immunity. “We’re turning the tide,” said Cohen. Parents are urged to consult pediatricians now—RSV season peaks in January.
Broader implications include policy shifts: Bipartisan bills seek permanent VFC inclusion, while WHO eyes nirsevimab for low-income nations. As U.S. wards breathe easier, the focus shifts to sustained vigilance against RSV’s annual threat.